Embark on a journey into the realm of chemical reactions and unravel the mysteries of predicting their products with Predicting Products of Chemical Reactions Worksheet Answers. This guide delves into the intricacies of various reaction types, empowering you with the knowledge and tools to anticipate the outcomes of chemical transformations.
Through a comprehensive exploration of reaction mechanisms, step-by-step tutorials, and practical applications, this resource equips you to navigate the complexities of chemical equations and master the art of predicting reaction products.
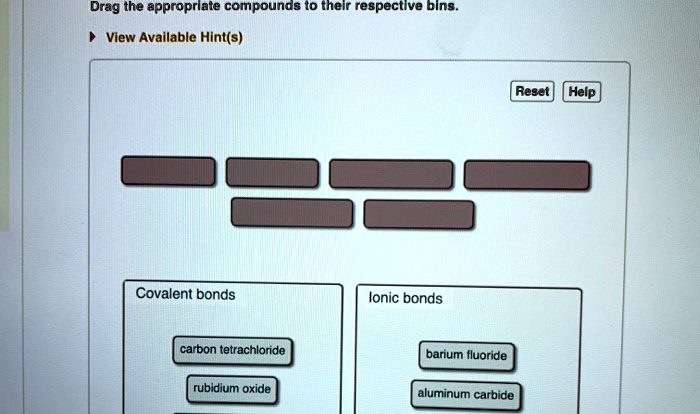
Types of Chemical Reactions
Chemical reactions involve the rearrangement of atoms and molecules to form new substances. Different types of chemical reactions exist, each with its unique characteristics.
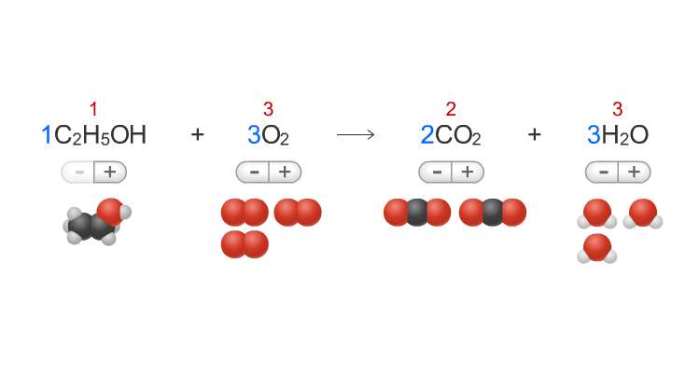
- Combination reactions: Two or more substances combine to form a single product. Example: 2H 2+ O 2→ 2H 2O
- Decomposition reactions: A single substance breaks down into two or more products. Example: 2H 2O → 2H 2+ O 2
- Single-displacement reactions: One element replaces another element in a compound. Example: Fe + CuSO 4→ FeSO 4+ Cu
- Double-displacement reactions: Two compounds exchange ions to form two new compounds. Example: NaCl + AgNO 3→ AgCl + NaNO 3
Predicting Products of Chemical Reactions: Predicting Products Of Chemical Reactions Worksheet Answers
Predicting the products of chemical reactions is essential in chemistry. Methods include:
- Chemical equations: Use balanced chemical equations to determine the stoichiometry and products.
- Activity series: Use the activity series of elements to predict the reactivity and displacement of elements in reactions.
- Solubility guidelines: Use solubility guidelines to predict the formation of precipitates in double-displacement reactions.
Step-by-Step Guide:
- Identify the reactants and their chemical formulas.
- Determine the type of reaction based on the reactants.
- Use the appropriate method to predict the products.
- Write the balanced chemical equation for the reaction.
Query Resolution
What are the different types of chemical reactions?
Chemical reactions can be classified into various types based on their characteristics, such as combination, decomposition, single displacement, double displacement, and redox reactions.
How can I predict the products of a chemical reaction?
Predicting reaction products involves understanding reaction mechanisms, balancing chemical equations, and applying knowledge of chemical reactivity and periodic trends.
What is the importance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side equals the number of atoms of that element on the products’ side, maintaining the law of conservation of mass.